LuMind IDSC and Other National Groups Urge CMS to Include DS Community in Alzheimer’s Drugs Coverage

August 18, 2021 LuMind IDSC has joined the National Down Syndrome Society (NDSS) and The National Task Group on Intellectual Disabilities and Dementia Practices (NTG) in sending comments to federal […]
A New Perspective on The Aduhelm Controversy

People with Down syndrome live longer and more independently now than ever before. Supported by advances in medical care, community programs, and supportive caregiving, people with Down syndrome are leveraging […]
LuMind IDSC signs on to NTG statement re: Aduhelm

“It is unknown to what degree aducanumab (Aduhelm) may help people with Down syndrome, as they were not included among the trial participants…” As the conversation around Biogen’s Aduhelm continues, […]
Why Patient Voices Are So Critical to the Development and Initial Approval of New Drugs
June 29, 2021 This blogpost from a former FDA insider on the power of the patient helps us understand how drug approval processes are informed by the patient experience, and […]
FDA Approves Biogen’s Aduhelm, the First New Drug for Alzheimer’s Disease Since 2003
Burlington, MA, June 7, 2021 – Today marks an important milestone in Alzheimer’s disease research with the approval by the FDA of Aduhelm (aducanumab) from the company Biogen. Aduhelm is the […]
Cross-Sectional Exploration of Plasma Biomarkers of Alzheimer’s Disease in Down Syndrome: Early Data from the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study

May 13, 2021 LuMind IDSC’s Chief Scientific Officer, James Hendrix, and a team of dedicated researchers and clinicians have published a key manuscript from the Down Syndrome Clinical Trial Network […]
LuMind IDSC Supported the T21RS COVID-19 Initiative’s International Survey on Patients with COVID-19 and Down Syndrome

May 3, 2021 LuMind IDSC supported the T21RS COVID-19 Initiative that brought about the original survey and the resulting analysis, which was recently published in The Lancet’s EClinical Medicine Journal. We […]
Findings Released for Ongoing Study of Clinical Trial Participation by the Down Syndrome Community

March 9, 2021 (Burlington, MA): LuMind IDSC Foundation, the National Down Syndrome Society (NDSS), and pharmaceutical leader Eli Lilly and Company (Lilly) recently released findings of a study that identifies […]
LuMind IDSC is Excited About a New Publication in the Journal of Patient-Reported Outcomes

As life expectancy of people with Down syndrome (DS) increases, so does the risk of Alzheimer’s disease (AD). Identifying symptoms and tracking disease progression is especially challenging whenever levels of […]
LIFE-DSR Natural History Study Blood Biomarker Data Shared at CTAD
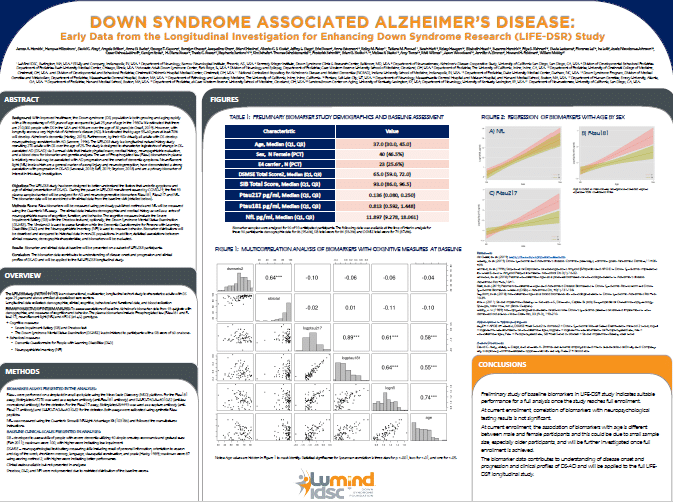
The yearly conference brings together leaders throughout the world who are dedicated to advancing treatment options for Alzheimer’s disease (AD). This year, Jim Hendrix presented the LIFE-DSR blood biomarker data […]
